Impact Assessment of COVID-19 Outbreak on Atopic Dermatitis Drugs Market
Exponential Growth of Biologic Drugs Favor Market Growth
According to a study published by BioMed Central, U.K – an electronic-only medical journal, it was found that, on a global level, ~15 – 20% children and ~1 – 3% adults suffer from atopic dermatitis (AD). The growing prevalence of this disease has triggered the demand for efficacious drugs in the atopic dermatitis drugs market.
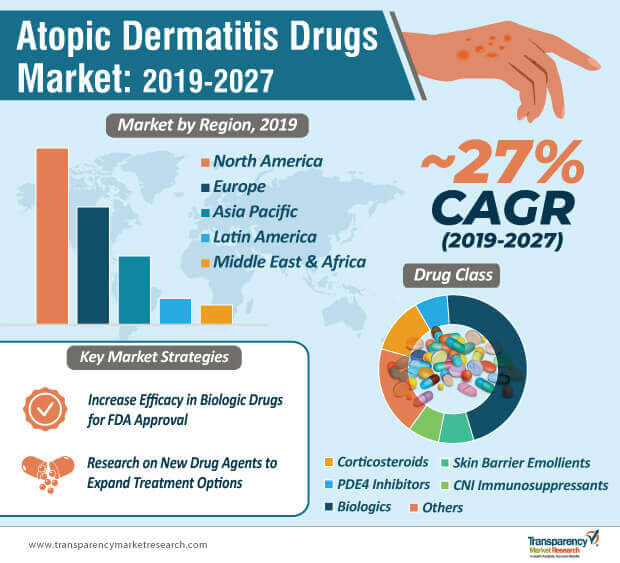
Biologic drugs are projected for exponential growth during the forecast period. In 2018, biologic drugs accounted for the highest market share of ~42%, and continue to dominate the market share with a projected value of ~77% in 2027. Thus, it is evident that, healthcare companies in the atopic dermatitis drugs market are increasing research & development activities to improve the efficacy of biologic drugs in order to provide instant relief for patients.
Since atopic dermatitis has a high risk of recurrence, healthcare companies in the atopic dermatitis drugs market are identifying drugs that improve the functioning of the immune system by helping to fight off bacteria and viruses. For instance, in August 2019, Sanofi – a French multinational pharmaceutical company, announced the approval of Dupixent® (dupilumab) – a biologic drug to treat moderate-to-severe atopic dermatitis, by the European Commission.
Request the Coronavirus Impact Analysis on this Market – https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=73639
To know the scope of our report Get a Sample on Atopic Dermatitis Drugs Market
Topical PDE4 Inhibitor Drugs Emerge as Non-steroid-based Option
Healthcare companies in the atopic dermatitis drugs market are developing new drug agents to expand the treatment options for the disease. They are developing new moisturizers that confer skin-barrier protection, biologics, and lipid-replenishing topical drugs. Manufacturers are making these treatment options available at retail and hospital pharmacies.
After several clinical trials, healthcare companies are introducing efficacious ointments that provide result-oriented outcome in patients. In 2027, PDE4 inhibitor (Phosphodiesterase Type 4 inhibitor) drugs will hold the second-highest revenue after biologic drugs in the atopic dermatitis drugs market. On that account, healthcare companies are propelling the use of nonsteroidal topical ointments that prevent the release of cyclic adenosine monophosphate (cAMP), which is one of the major causes that aggravates atopic dermatitis.
Buy this Research Report Now – https://www.transparencymarketresearch.com/checkout.php?rep_id=73639<ype=S
Stakeholders in the atopic dermatitis drugs market are gaining the faith of patients, as nonsteroidal topical ointments are considered safer and better than topical corticosteroids, especially for younger patients. Due to the high prevalence of atopic dermatitis in children, healthcare providers in the atopic dermatitis drugs landscape are observing a shift in consumer preference, as caregivers are hesitant to opt for steroid-based options.
Experimental Biologics Reduce Side Effects of Atopic Dermatitis Drugs
Although topical treatment drugs are projected for growth in the atopic dermatitis drugs market, in many cases, these treatments have been ineffective in many patients. As a result, patients are advised to opt for traditional immunosuppressant medications such as cyclosporin (CYA) and methotrexate (MTX). These immunosuppressants show effectiveness in AD, but their routine use is demonstrated with incompetent disease responses and other adverse effects in patients. Also, there is a risk of renal, hepatic, and other toxicities in body organs that adversely affect the healing period of these treatment options.
Thereupon, healthcare companies are approaching experimental biologics to prevent the toxicity and improve the efficacy of atopic dermatitis drugs. They should focus on preventing skin inflammation and flares while minimizing the side effects of these drugs.
More Trending Reports by Transparency Market Research – https://www.biospace.com/article/chlamydia-infection-diagnostics-and-therapeutics-market-nucleic-acid-amplification-tests-to-remain-most-preferred/

