IV Bags Market Growth Sales Revenue Analysis 2026
IV Bags Market: Introduction
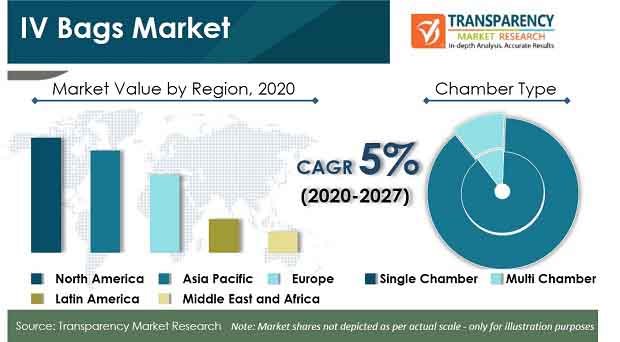
According to the latest market report published by Transparency Market Research titled “IV Bags Market: Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2020–2027,” single chamber IV bags segment is expected to be the largest contributor to the growth of the global IV bags market during the forecast period. Globally, the revenue generated from sales of IV bags in global market has been estimated to be ~US$ 1.6 Bn in 2020, and is projected to increase at a CAGR of 5% during forecast period 2020-2027.
IV bags are an ideal means for the transportation of nutrients and other nutritious compounds for patients who cannot ingest anything through the oral route of administration. IV bags mare made of plastic films and produced in a variety of capacities such as 0-250 ml, 250-500 ml, 500-1,000 ml, and above 1,000 ml.
Request For Covid19 Impact Analysis Across Industries And Markets @ https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=23147
IV bags have emerged as a crucial element of growth for the parenteral nutrition market where they are highly preferred for intravenous administration of compounds such as amino acids, etc. Rising ageing population is also contributing to the growth of the global IV bags market. Ageing brings along various ailments, thereby giving rise to the demand for efficient drug administration methods. IV drugs are preferred to oral drugs, as the former generates results in less time and has higher accuracy. Rise in awareness among consumers is expected to directly translate to preference for IV administration, thereby fueling the growth of the market during the forecast period.
The global IV bags market is expected to expand at a high CAGR of 5% during the forecast period, 2020-2027, owing to factors such as rise in adoption of injection-based therapies and increasing prevalence of chronic diseases. The growth of the IV bags is marred by increased risk of leachable and extractable, which get potentially exposed to the solution inside the IV bags after dilution of therapeutics. Therefore, it becomes crucial to keep track of the compatibility of the therapeutics in IV bags.

