World’s only sample of Metallic Hydrogen has escaped in the Harvard Laboratory
World’s first ever piece of metallic hydrogen that was created last month has vanished from the Harvard Laboratory. Scientists say that the precious metal disappeared due to a sudden change in pressure. However, the research team has reassured that soon they will be able to revive the metallic hydrogen again.
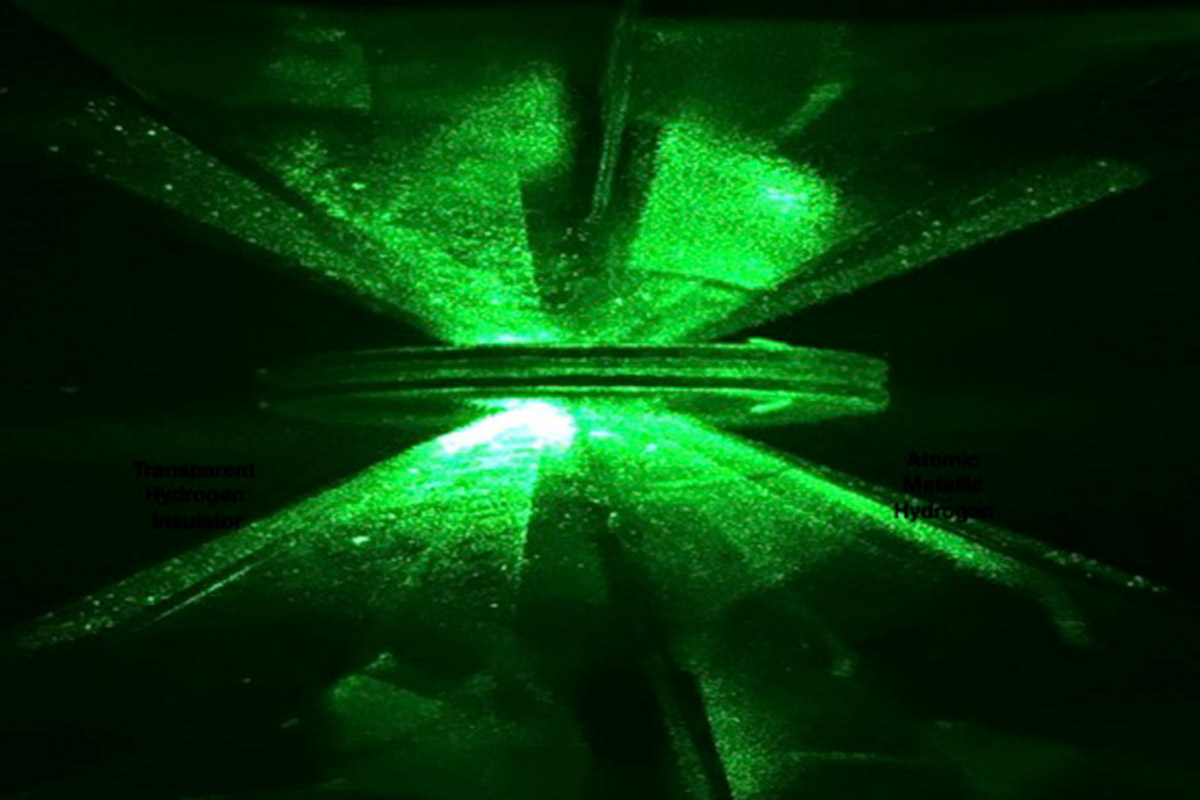
A team of scientists at Harvard University converted an eight-decade-long theory into reality when they made metallic hydrogen in the lab. The astonishing feat was considered impossible by many physicists due to the chemical composition of the lightest element in the universe — hydrogen. However, a team led by Isaac F Silvera kept hydrogen atoms under high pressure between two diamonds which resulted in the formation of first ever sample of metallic hydrogen measuring 1.5 micrometres thick and 10 micrometres in diameter.
Apparently, the world has lost the only piece of hydrogen in metallic form. Researchers explained that preventing the element from decaying was a very cumbersome task as it needed very low temperature and high pressure. Scientists kept the sample at minus 193 degrees Celsius and applied extremely high pressure, but somehow, the pressure dropped which forced the hydrogen molecules to expand and escape into the atmosphere.
While giving the statement, Silvera said that the molecules disappeared either into the room or just turned into gas, and we have lost the only sample of metallic hydrogen. However, his team is motivated to make another sample of most precious metal on Earth under high pressure and low temperature.
To create the sample, researchers used a DAC (diamond anvil cell) capable of withstanding pressures as high as 495 GigaPascals and lowered the temperature till 3 Kelvin. Scientists kept hydrogen atoms between the diamond anvil and found that hydrogen started displaying metal-like properties at such low temperature and high pressure. Further increasing the pressure resulted in the appearance of solid molecular substance with a shiny surface. It was the first ever sample of metallic hydrogen the world ever saw.
However, the research team at Harvard didn’t stop there and further decreased the temperature which eventually turned the metal black signifying that the substance has semiconductor-like properties and can absorb light.
However, some researchers are sceptical over the process of making of metallic hydrogen and believe that the team took only one precise measurement of pressure which will make it tough to know when actually pressure deviated from being constant and helped hydrogen molecules in escaping.
“This disappearance doesn’t say anything about the validity of the sample. Anyone who does high-pressure works knows that you have failures like this,” Silvera said.


